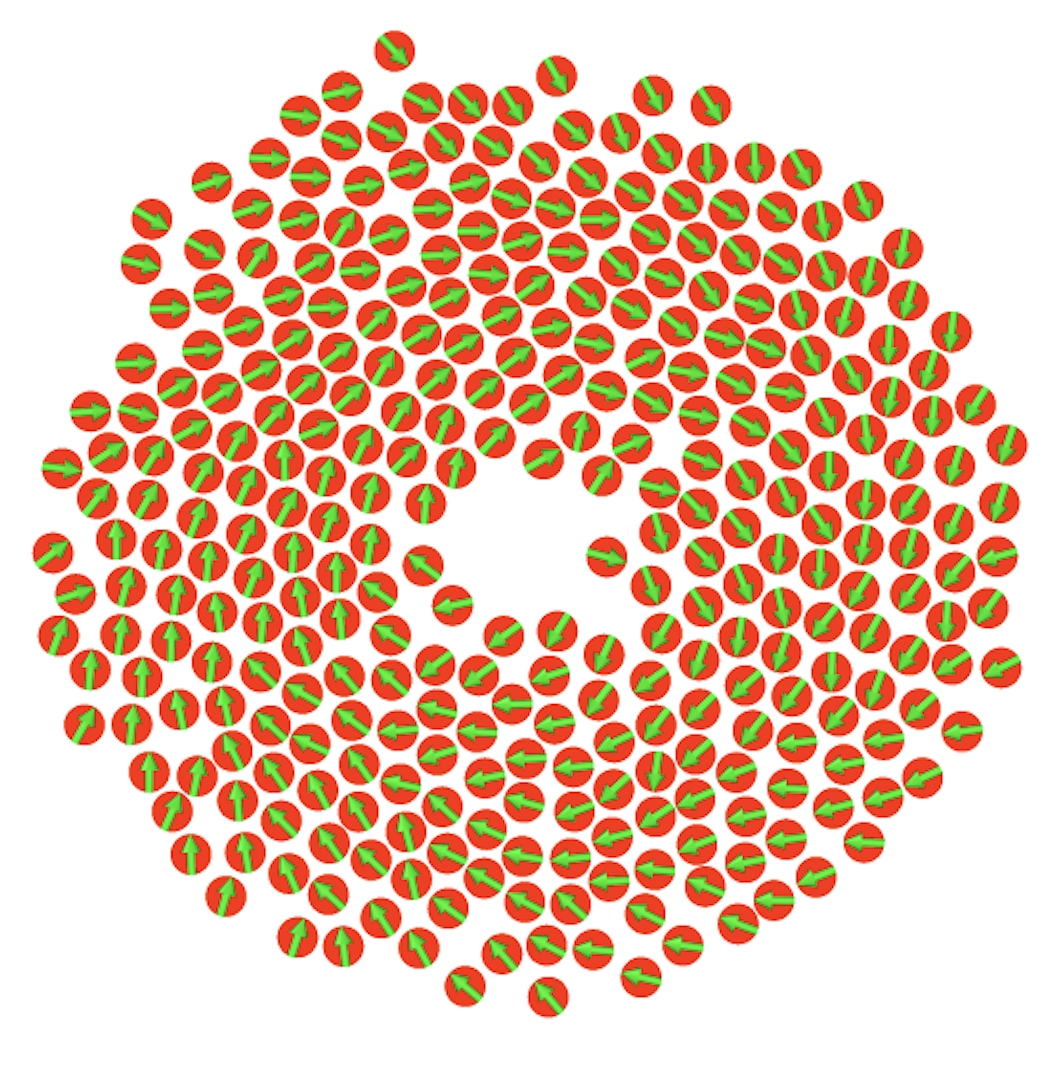
Packing of a helical polymer into a capsid
Torsional elasticity strongly shapes how a semiflexible helical polymer packs into
and ejects from a confined capsid. Mild torsional rigidity speeds up packing,
while higher stiffness first slows and then accelerates it due to competition
between spooling and increased persistence length—a feature that disappears without confinement.
Torsional stiffness also promotes spool-like structures, whereas ejection slows monotonically as rigidity impedes uncoiling.
Overall, torsional mechanics critically influence both packing and release in confined polymers such as viral DNA.
References: J. Chem. Phys. (2025);
Adv. Theory & Simulations(2025)